Flow Cytometry Facility
The SOM Flow Cytometry Facility (SOMFlow) provides cell analysis and sorting to Deakin researchers, as well as the scientific research community based at Geelong.
SOMFlow offers access to flow cytometry equipment and expertise that enables analysis, isolation and separation of cells of interests from mixed populations. The ability to separate and obtain cells of a particular type greatly enhances the scope of further detailed analysis.
SOMFlow offers comprehensive training and education, experimental design and protocol guidance specifically targeting effective data generation and interpretation in the field of flow cytometry. Our PC2 facility currently houses one BD FACSCantoTM II analyser (3-Laser, 8-Color) and one BD FACSAriaTM III sorter (4-Laser, 12-Color). Training is provided for analyser, and analyser is operated by qualified and licensed users. Cell sorter is operated by the core that provides services catering for both animal and human cell sorting in a PC2 environment.
All users must be familiar with the booking rules, associated usage costs, usage guidelines and standard operating procedures (SOPs) (DOCX, 66.5KB). Please complete the Use and Training Request form (PDF, 599.0KB) along with reading the SOP's. For more information please do not hesitate to contact us.
Dr Bing-Ru Wu
+61 3 522 78593
bingru.wu@deakin.edu.au
Prof John Stambas
+61 3 52275740
john.stambas@deakin.edu.au
Hourly rate
Instrument | Licensed User Operated | Core Operated | ||||
|---|---|---|---|---|---|---|
SOM | Deakin | External | SOM | Deakin | External | |
FACSCanto II analyser | $30 | $40 | $50 | |||
| LSR Fortessa X20 | $30 | $40 | $50 | |||
FACSAria III cell sorting service | - | - | - | $80 | $90 | $120 |
Booking conditions and policy
SOM Flow Cytometry Facility (SOMFlow)
For all enquiries regarding the Flow Cytometry equipment, please contact us.
FACSCanto II – PC2 Facility
The analyser is available for researchers to operate 24/7 following training and reaching a high level of competence. For training and assisted usage please book.
Bookings can be made online via Outlook calendar using these Instructions.The calendar names are *G HEALTH Medicine Lab Eqpmt ka4.308 SOMFlow and *G HEALTH Medicine Lab Eqpmt ka4.327 FlowJo Dongle
Minimum booking is 1 hour, followed by 30 minute increments thereafter. Bookings are essential.
All cells must be sieved through 40 µm mesh prior to analysis.
Charging starts from the time booked, so it is in your interest to arrive on time.
Cancellation policy
Cancellations can be made via the online booking system with between 24-48 hours’ notice.
Cancellations with less than 24 hours’ notice will be charged 50% of the booked time, minimum of 1 hour, and no-shows will be charged in full.
FACSAria III Sorter– PC2 Facility
The Sorter will be operated by the core. It is important to plan a meeting to discuss your sorting needs before experiments. Please contact us for availability.
Three sizes of nozzles are available: 70, 85, and 100 μm.
The rule of thumb when sorting is to use a nozzle that is 4 times larger than your cell size. If you want to sort lymphocytes (around 10 μm) then a 70 μm nozzle is used (this is the smallest size). Larger cells, such as HeLa (~20 μm) need a 100 μm nozzle. Selecting the correct nozzle size will ensure sorting efficiency, reduce cell death and prevent blockages. However, the larger the nozzle size, the slower the sorter can sort, and therefore, the fewer cells can be sorted during your booking.
Booking can only be made by contacting us.
Please indicate if you are sorting primary human tissue or other PC2 classified cells.
Yeast will not be accepted for sorting.
All cells for sorting must be sieved through 40 µm mesh prior to sorting.
**Please be aware that your booking time includes 20 min for machine set up, and 10 min after-sort cleaning, and an additional 5 - 10 mins if you require purity checks after sorting.
Minimum booking is 1 hour, after which users will be charged in 30-minute increments. Users will be charged based on the time booked or time used, whichever is longer.
Cancellation policy
Users can cancel their bookings by contacting us up to 24 hours before their sort. Cancellations with less than 24 hours’ notice will be charged 50% of the booked time, and no-shows will be charged in full.
Training is essential and compulsory for using the analyser. Please fill in the SOM Flow Cytometry Use and Training Request Form prior to use and training for the facility. For training to become a licensed user please e-mail us
Training fees
| Trainee | Rates |
|---|---|
| School of Medicine | $100 |
| Other Deakin students | $110 |
| External users | $120 |
*Training fee is a one-off payment
Step 1: Online tutorials
It is essential for users to watch these tutorial videos prior to face-to-face training.
- Flow Cytometry Tutorials from Life Technologies.
- Compensation: An informal perspective - from Mario Roederer, co-author of flow analysis software Useful reading for understanding what compensation is.
- Purdue University Cytometry Lecture Slides by J. Paul Robinson, a senior figure in the flow cytometry world.
Step 2: SOMFlow Online Quiz
Once you complete the quiz your results will be automatically sent to the SOMFlow team, who will be in touch with the outcome of your result.
Step 3: Group induction
4 people per group.
Please email a request for your group induction training session.
For students who wish to organise a group induction for the FACSCanto II and a follow-up first trial run of flow cytometry, please discuss with and obtain an approval from your supervisor(s) first and forward the approval to me. This is to ensure that your supervisor(s) are aware of your intention and can decide whether it is necessary for your project.
If there are multiple people from your group would like to get trained, please arrange a time among yourselves before booking so that all of you (no more than 4 people) can attend in the same session.
Please be advised that flow cytometer training should be taken no more than 3 weeks prior to your actual experiments. This is to ensure that you can utilised the new skill as soon as possible to become competent and independent quickly.
You can organise the final licensing step after this training session.
Step 4: One-on-one licensing
This step should be arranged as a trial run of your actual experiment using experimental samples (eg. cells) and include proper staining controls (eg. unstained control, single colour control). This step should be arranged no more than 3 weeks after the group induction.
Please discuss with us to arrange a suitable time and then make the online booking.
This section requires you to be able to :
- perform fluidics start-up
- navigate the acquisition software BDDiva, generate worksheets and set gates
- set baseline voltages
- compensate using single colour controls
- clean and shutdown the instrument.
You are considered 'licensed' when these steps are performed correctly without any help.
You will then be added to our licensed user list so that you can book the analyser and commence your flow experiments on your own.
Educational resources
Online tutorials
- Flow Cytometry Tutorials from Life Technologies.
- Flow Cytometry Tutorials from BD.
- Compensation: An informal perspective - from Mario Roederer, co-author of Flow Analysis software Useful reading for understanding what compensation is.
- Purdue University Cytometry Lecture Slides. By J. Paul Robinson, a senior figure in the Flow Cytometry world.
- Excyte - Modern Flow Cytometry Handbook (Antibody Panels, Data Analysis, Controls, Cell Sorting, Statistics)
Instrument user guide
BD FACSCanto II Flow Cytometer Reference Manual
BD FACSDiva Software 6.0 Reference Manual
Multicolor tools
Reagent selection guide for multicolor flow cytometry
- BD Multicolor Flow Cytometry Application Note

FACSCanto II
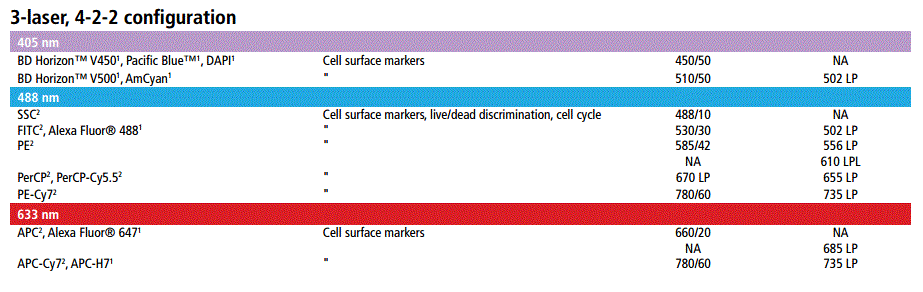
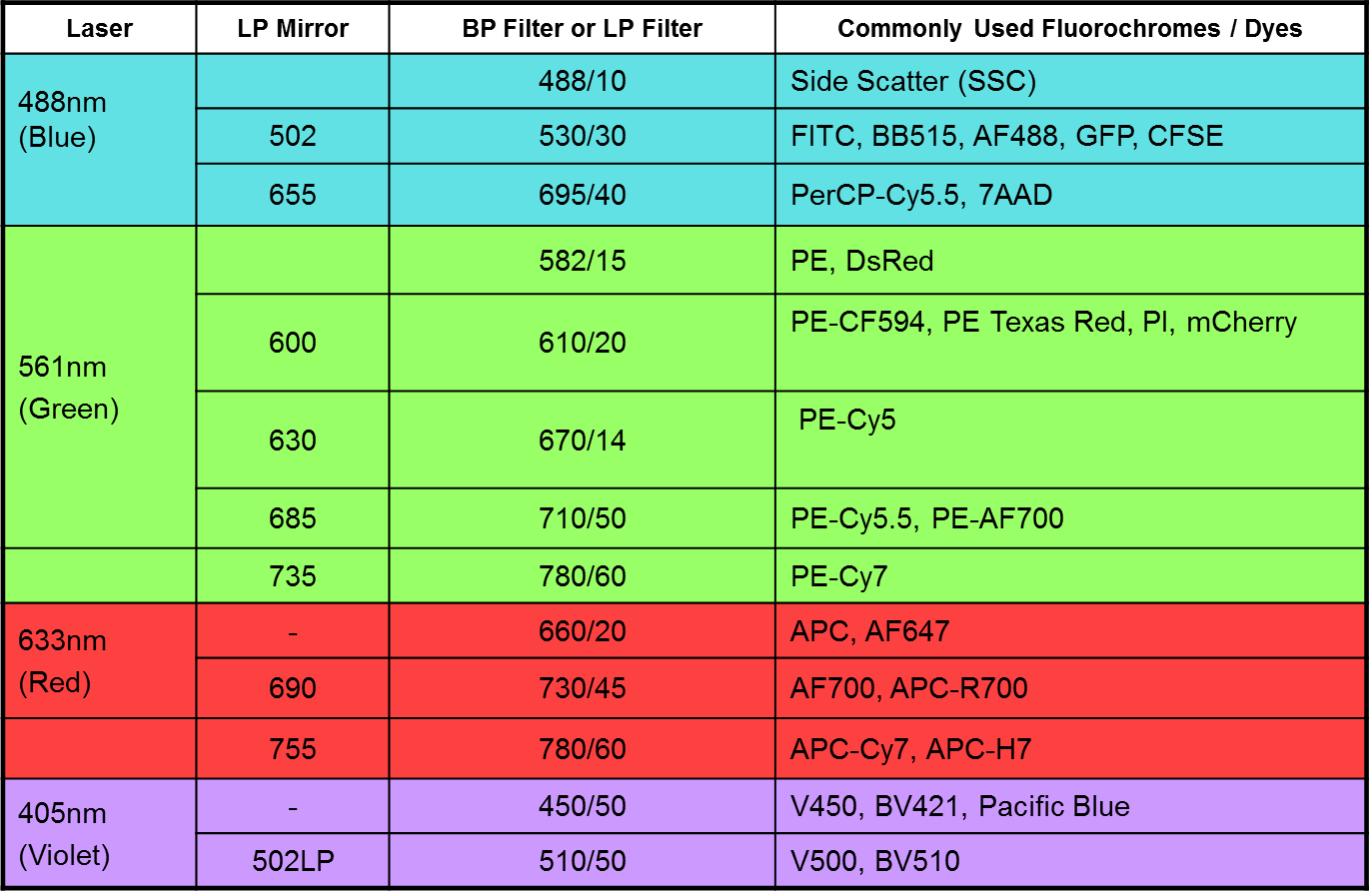
- A 3-laser 8-Color system (Violet/Blue/Red; 405 nm, 488 nm and 633 nm)
- Tubes: BD Falcon™ tubes 12 x 75-mm
- Analysis Rate: Maximum 10,000 events per second


FACSAria III
- Automatic Cell Deposition Unit (ACDU) allows for sorting into 6, 24, 48, 96, and 384-well plates, and onto slides
- Collect up to 4 populations for a variety of tube sizes (12 x 75-mm, 15 ml and 1 ml microtubes)
- Sterile Sorting
- 70, 85 and 100-micron nozzle tips
- Maximum acquisition rate: 70,000 events per second
- An assay that has been optimized on the BD FACSCanto II Flow Cytometer is easily transferable to the BD FACSAria III without loss in signal resolution. Applications developed on the BD FACSCanto II will look similar when transferred to the BD FACSAria III.
- A 4-laser (Violet/Blue/Yellow-Green/Red; 405nm, 488nm, 561nm and 633nm), 12-Colour system:
BD FACSCanto II
Q: How do I decide which fluorochromes to use?
A: Always use fluorescent dyes and proteins with as little spillover as possible between them. To achieve minimal fluorescence spillover, use fluorochromes which are excited by different lasers, eg. a good basic four colour combination would be:
Blue laser - FITC
Violet laser - Pacific Blue
Yellow-green laser - PE
Red laser – APC
Use fluorochrome combinations with as large a difference in emission wavelength as possible
(eg. PE-Cy7 or APC-Cy7 can be used with the fluorochromes listed above.
If only required a few colours in your assay, choose the brightest available fluorochromes:
PE > APC > PE-Cy7 > FITC > Pacific Blue
Do not use fluorochrome combinations with similar or identical emission wavelengths. Examples of incompatibility include: Pacific Blue and DAPI; RFP and PI; APC / Alexa647 and PE-Cy5; PE and
Alexa568 / 594.
For more information, please refer to the “Multicolor Tools” and “Reagent Selection Guide for Multicolor Flow Cytometry” listed on this website.
Q: What concentration of antibody should I use?
A. Always titrate each new vial of antibody even if you have used the same antibody before. Also titrate antibody under similar experimental condition that you will use during your experiments. This means using roughly the same number of cells per tube, same volume of stain, same length of time for staining, same method of washing, and choice of fixative.
Q: Why do I have to filter my cells immediately prior to being run on FACSCanto II and FACSAria III?
A. This is to avoid cell clumping, which has the potential of causing blockages in the fluidics of the flow cytometers. Choices of cell strainers are nylon mesh strainers: 40 um; 70 um; 100 um. These fit into 50 ml centrifuge tubes and are good for filtering cells in bulk at beginning of experiment if you have a large volume
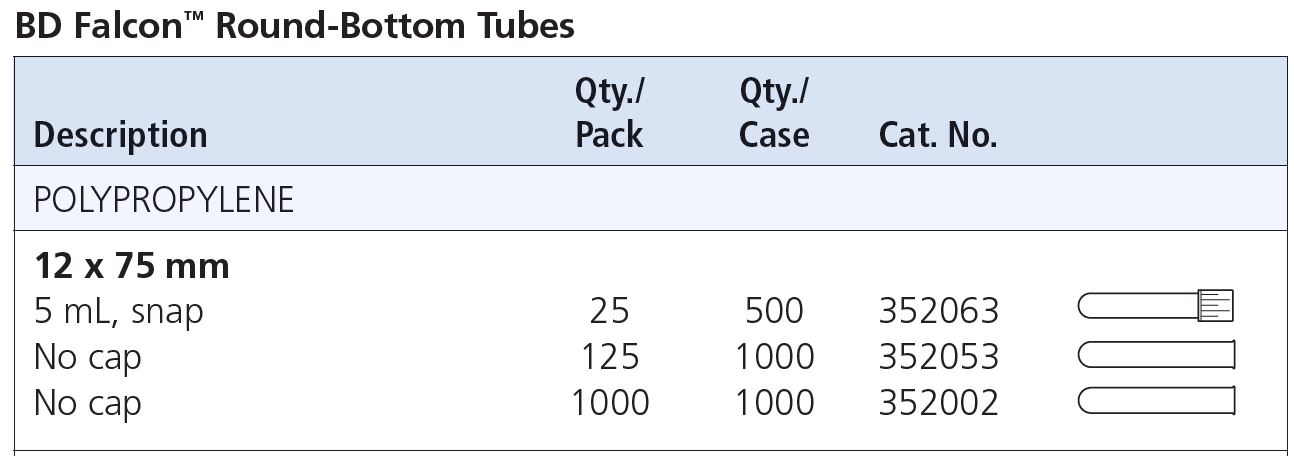
Q: What kind of FACS tubes should I use?
A: 12x75 mm round-bottom tubes.
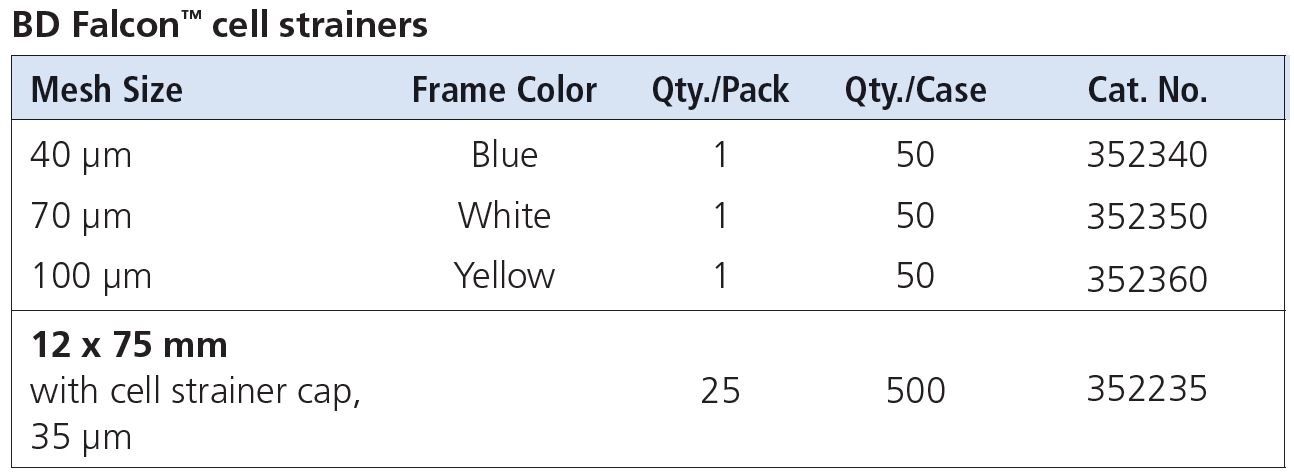
Q: What kind of cell strainers should I use?
A: There are several options for cell strainers.
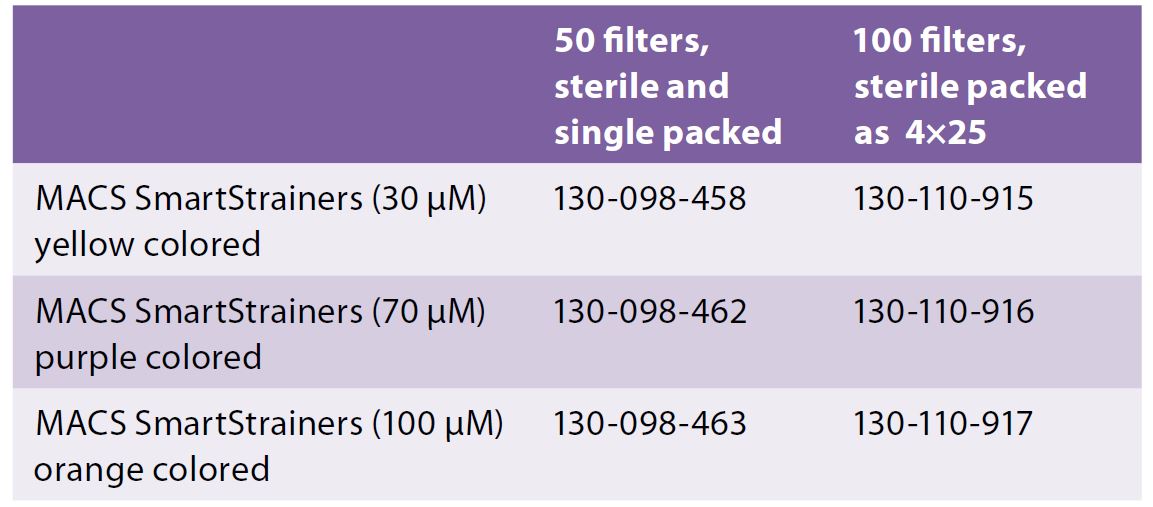
For tissue processing, you can use Cell Strainers (40, 70, 100 μm nylon mesh) from BD (FalconTM cell strainers) or Milteny Biotec (MACS SmartStrainers) that can be fitted onto a 50mL conical tube to isolate primary cells and obtain single cell suspensions prior to analysis.
For cells already in suspension, use BD Falcon 12 x 75 mm Tube with Cell Strainer Cap (Cat. No. 352235) that has a 35 μm nylon mesh incorporated into the tube cap to dissociate and collect samples for downstream processing in cell analysing or sorting instruments.
Q: Which software can I use for flow data analysis?
A: You can use BDDiva for simple data analysis. We recommend to use FlowJo for comprehensive data analysis and graphing for publication purposes. You can sign up for a 30-day free trial. SOMFlow also has a FlowJo dongle available for booking. To book a dongle, please go to “Overview”à “Booking Policy” for instructions.
Q: Which controls do I need?
A: Unstained cells, single color controls for each fluorochrom, experimental controls if necessary (positive or negative controls), live/dead staining
Q: How much time should I book for FACSCanto II?
A: Minimal 1 hour. This takes into consideration the time it takes to do fluidic start-up and shut-down, change sample tubes. It always takes longer than you think.
BD FACSAria III
Q: How long should I make my appointment for?
A: Minimal 1 hour. Sorting time depends on several factors, including the starting cell number, percent of target cells, required target cell number, sort purity requirement, nozzle size.
Q: How many cells should I bring?
A: Minimal starting cell number is 1 x 106. Usually, for 100 µm nozzle: 5 - 7 x 106 cells/mL; for 70 µm nozzle: 20 - 30 x 106 cells/mL. Cells for sorting should be in 0.5 mL minimum volume if you have less than 1x106 cells.
Q: How many cells will I get back?
A: “Worst case” – lose 50% of the possible target cell number due to cell death during and after sorting.
Q: Which controls do I need?
A: Unstained cells, single color controls for each fluorochrom, experimental controls if necessary (positive or negative controls), live/dead staining
Q: Which nozzle size should I request?
A: cell size ≈ 1/5 of nozzle size. In general, use 70 µm nozzle for suspension cells; use 100 µm nozzle for adherent cell lines and cells from tissue dissociations
Q: Which “sort precision mode” should I choose?
A: Purity (high purity) vs. Yield (high recovery) vs. “Single” (count accuracy + purity)
Q: What are good sorting buffers?
A: A basic receipt is PBS (Ca/Mg2+-free) + 1mM EDTA + 25mM HEPES, pH 7.0 + 1% -10% FBS (Heat-inactivated). Optional: add 10U/mL DNAase II to remove genomic DNA if you have lots of dead cells in your sample. Culture media is not suitable!
Q: What are good collection media? A: contains HEPES and high concentration of serum (5-10%FCS)
For more information, please see Sample Preparation Guide for BD FACSAria Cell Sorter.
